Primary Standard of Emf (Saturated normal Weston cell )
The saturated normal Weston cell is known as the primary standard voltage source developed by Edward Weston in 1892.
Note: this is a voltaic cell.
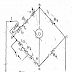
Construction and Characteristics: the construction shown in figure
Positive electrode: Mercury
Negative electrode: An amalgam of cadmium which is 1 part of cadmium and 7 parts of mercury.
Electrolyte: Saturated solution of cadmium sulphate.
Depolarizing agent: A paste of mercurous sulphate Hg2So4 is placed on top of mercury.
Note: An excess of solid crystals of CdSO4.8H2O ( cadmium sulphate) in the electrolyte space and the depolarizer assures that the solution will be saturated at all temperatures.
A simplified cell reaction is :
-The emf of the cell remains constant as long as amalgam has both solid and liquid phases are present.
-The Weston cell is contained in an H- Shaped vessel.
-The limbs of the H vessel are hermetically sealed and the connections to the external circuit are made through platinum wires.
-Sulphuric acid is added to act as an electrolyte.
-Saturated cells are normally allowed to age for a year before they are considered to be of constant emf. Following this period, their life appears indefinite excluding the possibility of accidents.
The emf of the cell is 1.01864 volt.
The emf of the cell changes with temperature and the equation relating emf to temperature is :
The cell has a long life if handled carefully. It has a life span of about 10 to 20 years. The drift in value of voltage is about 1 microvolt per year.
Note: The cell is a standard for maintenance of the volt and as such, it is only used in standardizing laboratories. Saturated cells are not used for ordinary work because of temperature requirements and also because they are not portable. The unsaturated cell is used for ordinary measurments.
Precautions:
1. Mercurous sulphate is sensitive to light and therefore it should not be exposed to light .
Note: Exposure to an incandescent light at ordinary levels of illumination is much less serious than direct sunlight or even light from the usual fluorescent lamps.
2. It has been mentioned above that the cell has a temperature coefficient of about -40 microvolts per degree Celcius. In that, it has an effect of +310 microvolts per degree Celcius at the positive terminal and -350 microvolts per degree Celcius at the negative terminal.
Therefore it is important to keep the entire cell at a uniform temperature to secure as much cancellation of positive and negative effects. Hence these cells always used in close temperature regulation, usually an oil bath held constant to better than 0.01 degree Celcius.
3. Saturated cells should be kept in an upright position because if they are tilted there is always the danger of mixing of electrode materials. Therefore these cells cannot be shipped and are sent through special messengers.
4. The principal limitation of the use of a standard cell is the current drawn from the cell at any time. This should be negligibly small. Even this small current should be allowed to flow for a very short period of time. It should be understood that standard cells are potential devices and will be damaged if the current is drawn from them.
it is difficult to measure the maximum value of current drawn from a standard cell since damage to the cell is a function of both magnitude and duration of current flow. The makers specify the maximum value as 100 microamperes but this should be regarded as an extreme figure. This means that the current drawn from the cell should be less than 100 microamperes and this current should flow momentarily.
A voltmeter should never be used for measuring the voltage of a standard cell. Firstly because of the current drain, there is a possibility of damage to the cell and secondly, this measurement is meaningless since the cells have a high internal resistance ( about 600 to 800 ). The voltage should be measured with the help of a potentiometer.
A cell should be handled with care and never be short-circuited. The excessive current causes a change in emf which may be permanent because the cell may not be able to recover. A cell once short-circuited, should be regarded with suspicion and hence is of little value as a standard.
When using the cell in potentiometric work, a resistance of 20,000 ohm should be inserted in series with the cell in order to avoid to an excessive drain of current when there is unbalance.
It is important that leakage resistance between the terminals of the cell be very high otherwise it may not give the standard voltage it is supposed to give.
Example1: A standard cell has a voltage rating of 1.018500 V and internal resistance of 500 ohm. The insulation resistance between its terminals is 5 Mohm
. Find the current drain due to insulation resistance. Calculate also the difference between the internal voltage and the terminal voltage.
Solution: Current flowing through the insulation resistance = (( 1.018500 )/(500+5x10^6 ) ) =0.204x10^-6 A
=0.204 micro Ampere
Thus there is a constant drain of a current of 0.204 micro Ampere.
Voltage drop = ( 0.204 x 10^-6 ) x 500
= 0.102 x 10^-3 V = 0.102 mV
Therfore , Difference between internal voltage and terminal voltage = 0.102 mV
Terminal voltage = 1.018500 - 0.000102
= 1.018398 V.




No comments:
Post a Comment